Services
- HOME
- Services
With passion and exceptional technologies,
experts in CMC research support the creation of outstanding and innovative drugs.
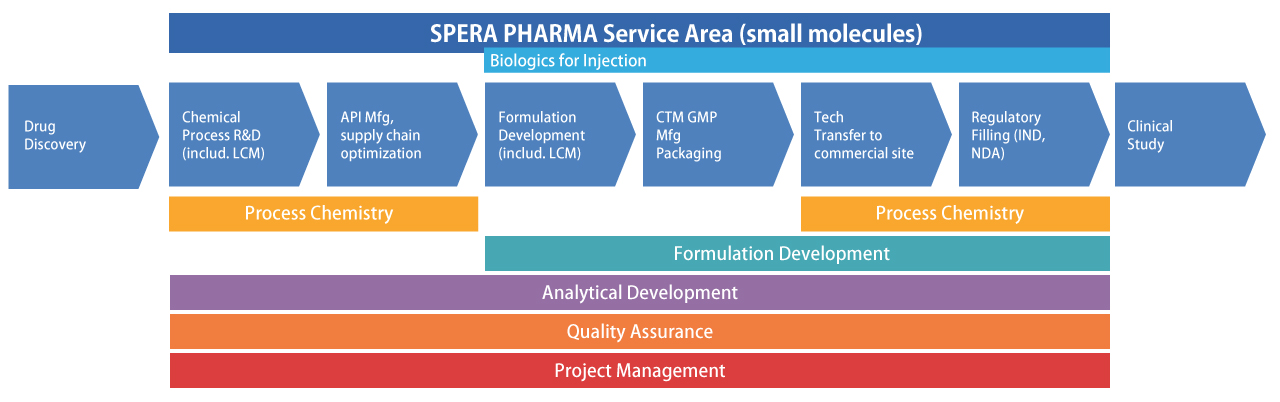
SPERA PHARMA was established as a spin-out of leading Japanese pharmaceutical company by Chemistry, Manufacturing and Controls (CMC) research experts, who were already responsible for delivering numerous new drugs to high global acclaim. SPERA PHARMA is responsible for the entire CMC process, from R&D through application; it provides services that meet the needs of customers. As the development of new drugs becomes increasingly difficult, SPERA PHARMA will hone technologies it has fostered and refined, advancing the research and development of promising drugs, and helping to achieve an even healthier society through the creation of outstanding new drugs.
page top