Technology
- HOME
- Technology
- High sensitivity analytical technology supporting impurity control
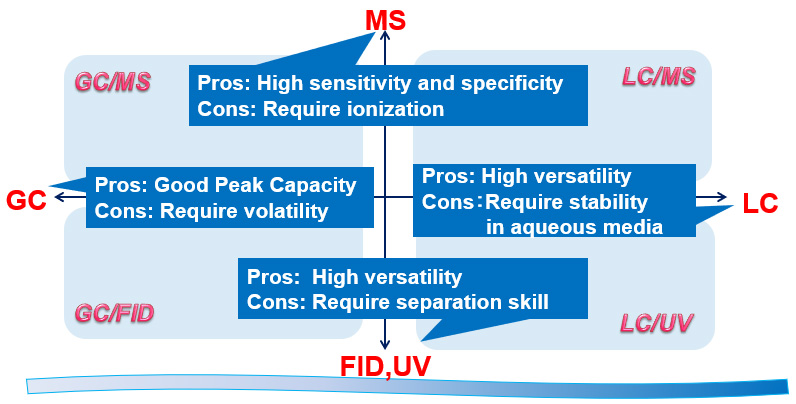
High sensitivity analytical technology supporting impurity control
In recent years, the regulatory authorities have focused on the impurities remaining in drug substances and they are demanding pharmaceutical companies to control them strictly. In particular, the controlled level of potential mutagenic impurities (PMIs) is often in units of ppm, but SPERA PHARMA uses highly sensitivity analytical techniques appropriate for each of the PMIs, which guarantees high quality for the drug substances. We have already established more than 300 analytical methods that guarantee the residual PMIs to be less than 10 ppm.
Methodology Matrix
Inquiries About Service
SPERA provides CMC solutions from early-stage through new drug application.
Contact USpage top