Technology
- HOME
- Technology
- FDC / Fixed dose combination (Dry-coated tablets)
FDC / Fixed dose combination (Dry-coated tablets)
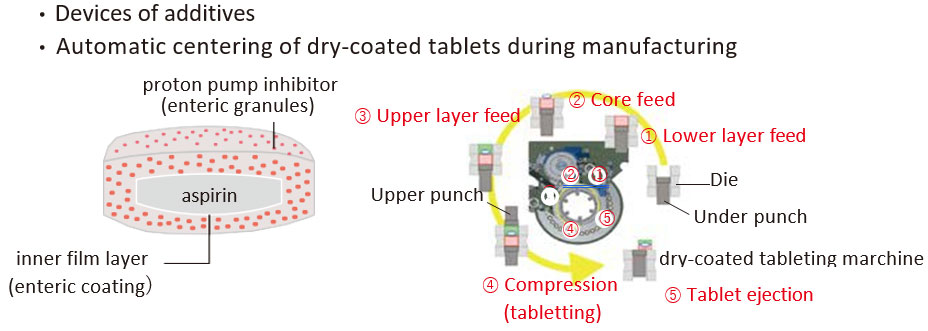
Success in complete separation and miniaturization of APIs: Development of formulation as a dry-coated tablet
Formulation design as a dry-coated tablet
- Complete separation to avoid interaction of the APIs
- Maintain the same formulation system of APIs as the single agent (BE)
- Miniaturization maintaining the strength of the formulation
A fixed dose combination of a proton pump inhibitor (Therapeutic agent for peptic ulcer) and a low-dose aspirin has been developed and launched as a FDC containing 100 mg of aspirin and 15 mg of a proton pump inhibitor in one tablet, and its usefulness including formulation characteristics is highly evaluated.
Inquiries About Service
SPERA provides CMC solutions from early-stage through new drug application.
Contact USpage top